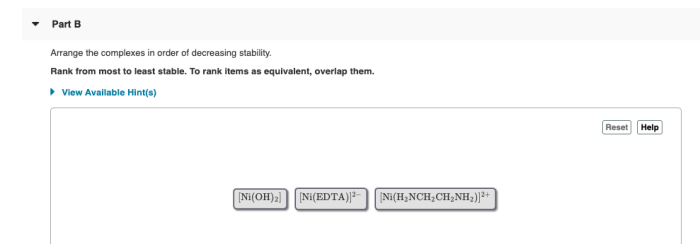
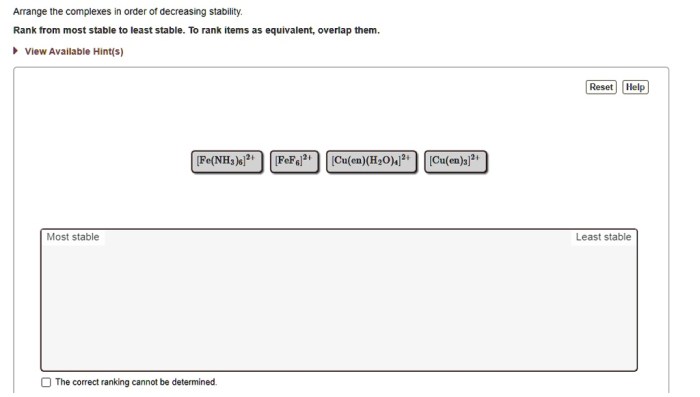
Arrange the complexes in order of decreasing stability – In the realm of coordination chemistry, the stability of complexes is paramount. This article delves into the intricacies of arranging coordination complexes in order of decreasing stability, exploring the factors that influence their stability and the methods used to order them.
Coordination complexes, formed when a metal ion binds to ligands, exhibit varying degrees of stability. Understanding the factors that contribute to stability is crucial for predicting the behavior and reactivity of these complexes in various chemical systems.
Coordination Complexes
Coordination complexes are chemical compounds that contain a metal center surrounded by ligands, which are molecules or ions that donate electrons to the metal. These complexes play a vital role in many biological processes, such as oxygen transport by hemoglobin and electron transfer in photosynthesis.
Examples of coordination complexes include:
- [Co(NH 3) 6] 3+(hexamminecobalt(III) ion)
- [Fe(CN) 6] 4-(hexacyanoferrate(IV) ion)
- [PtCl 4] 2-(tetrachloroplatinate(II) ion)
Stability of Coordination Complexes
The stability of a coordination complex refers to its resistance to dissociation into its constituent ions. Several factors contribute to the stability of coordination complexes, including:
| Factor | Explanation |
|---|---|
| Charge of the metal ion | Higher charge leads to stronger electrostatic attraction between the metal ion and the ligands, resulting in increased stability. |
| Size of the metal ion | Smaller metal ions have a higher charge density, leading to stronger electrostatic interactions and increased stability. |
| Nature of the ligands | Ligands with more donor atoms and stronger donating ability form more stable complexes. |
| Chelate effect | Ligands that can form multiple bonds with the metal ion create a chelate effect, which enhances stability by reducing the entropy of the system. |
Decreasing Stability: Arrange The Complexes In Order Of Decreasing Stability

Coordination complexes can exhibit decreasing stability due to various reasons, such as:
- Increasing temperature: Higher temperatures increase the kinetic energy of the molecules, leading to increased dissociation.
- Changing pH: Changes in pH can affect the charge of the metal ion and the ligands, altering the electrostatic interactions and stability.
- Competing ligands: The presence of other ligands in solution can compete with the original ligands for binding to the metal ion, reducing the stability of the original complex.
- Redox reactions: Redox reactions can change the oxidation state of the metal ion, affecting the stability of the complex.
Ordering Complexes
Complexes can be ordered in order of decreasing stability using various methods, such as:
| Method | Explanation |
|---|---|
| Thermodynamic stability constants | The equilibrium constant for the dissociation reaction of the complex provides a quantitative measure of its stability. |
| Kinetic inertness | The rate of dissociation of the complex can be used to compare its stability, with slower dissociation indicating higher stability. |
| Redox potentials | The redox potential of the metal ion can be used to assess the stability of the complex, with more positive redox potentials indicating higher stability. |
| Spectroscopic techniques | Spectroscopic techniques, such as UV-Vis and IR spectroscopy, can provide insights into the electronic structure and bonding of the complex, which can be related to its stability. |
It is important to note that the stability of coordination complexes can vary depending on the specific conditions and the methods used to measure it.
FAQ Resource
What is the significance of stability in coordination complexes?
Stability determines the complex’s ability to maintain its structure and composition under specific conditions, influencing its reactivity and applications.
How can we experimentally determine the stability of coordination complexes?
Stability constants can be measured using various techniques, including spectrophotometry, potentiometry, and calorimetry.