Organic and inorganic intermediate species play a crucial role in chemical reactions, serving as transient entities that bridge the gap between reactants and products. This article delves into the fascinating world of these species, exploring their formation, reactivity, and diverse applications across various fields.
Intermediate species, as their name suggests, are short-lived chemical entities that exist temporarily during the course of a reaction. They can be classified into two broad categories: organic and inorganic. Organic intermediate species contain carbon atoms, while inorganic intermediate species do not.
1. Organic and Inorganic Intermediate Species
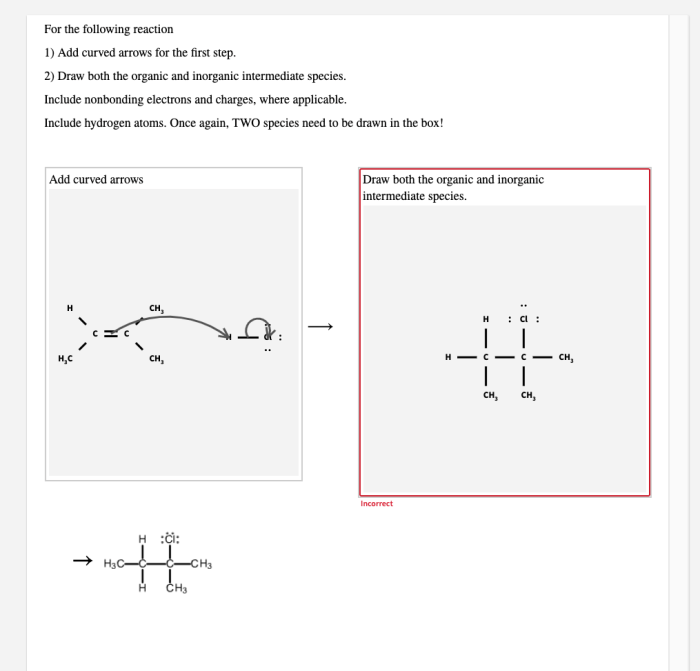
Intermediate species are short-lived chemical species that are formed during the course of a chemical reaction and are consumed before the reaction is complete. They play a crucial role in determining the rate and selectivity of chemical reactions.
1.1. Concept of Organic and Inorganic Intermediate Species
Organic intermediate species are species that contain carbon atoms, while inorganic intermediate species do not. Organic intermediate species are typically formed by the breaking of carbon-carbon or carbon-heteroatom bonds, while inorganic intermediate species are typically formed by the breaking of metal-ligand or metal-metal bonds.
1.2. Differences between Organic and Inorganic Intermediate Species
Organic and inorganic intermediate species differ in their structure, reactivity, and stability. Organic intermediate species are typically more stable than inorganic intermediate species, and they are more likely to undergo reactions that involve the formation or breaking of carbon-carbon bonds.
Inorganic intermediate species are typically more reactive than organic intermediate species, and they are more likely to undergo reactions that involve the formation or breaking of metal-ligand or metal-metal bonds.
2. Formation and Reactivity of Organic and Inorganic Intermediate Species
2.1. Formation of Organic and Inorganic Intermediate Species
Organic intermediate species are typically formed by the breaking of carbon-carbon or carbon-heteroatom bonds. The most common types of organic intermediate species are carbocations, carbanions, and radicals. Inorganic intermediate species are typically formed by the breaking of metal-ligand or metal-metal bonds.
The most common types of inorganic intermediate species are metal complexes and organometallic compounds.
2.2. Reactivity of Organic and Inorganic Intermediate Species
Organic intermediate species are typically more stable than inorganic intermediate species, and they are more likely to undergo reactions that involve the formation or breaking of carbon-carbon bonds. Inorganic intermediate species are typically more reactive than organic intermediate species, and they are more likely to undergo reactions that involve the formation or breaking of metal-ligand or metal-metal bonds.
2.3. Examples of Organic and Inorganic Intermediate Species
Some examples of organic intermediate species include carbocations, carbanions, and radicals. Some examples of inorganic intermediate species include metal complexes and organometallic compounds.
3. Role of Organic and Inorganic Intermediate Species in Chemical Reactions
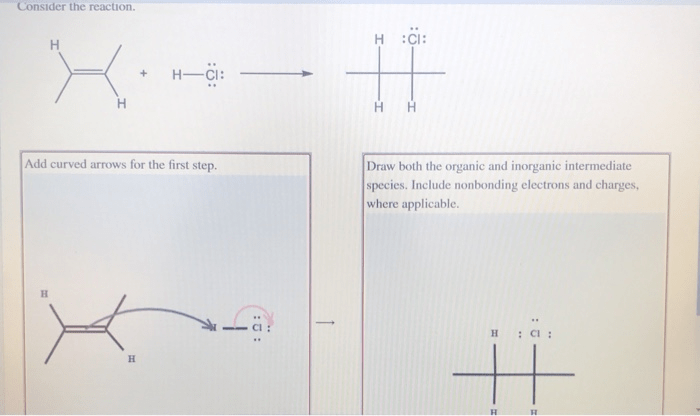
3.1. Role of Organic and Inorganic Intermediate Species in Chemical Reactions
Organic and inorganic intermediate species play a crucial role in chemical reactions. They can affect the rate and selectivity of chemical reactions, and they can also provide insights into the reaction mechanism.
3.2. How Organic and Inorganic Intermediate Species Affect the Rate and Selectivity of Chemical Reactions
Organic and inorganic intermediate species can affect the rate and selectivity of chemical reactions by providing alternative pathways for the reaction to proceed. For example, a carbocation intermediate can react with a nucleophile to form a new carbon-carbon bond, or it can rearrange to form a more stable carbocation.
The choice of pathway will depend on the relative stability of the two carbocations.
3.3. Examples of Chemical Reactions that Involve Organic and Inorganic Intermediate Species
Many chemical reactions involve organic and inorganic intermediate species. Some examples include the SN2 reaction, the E2 reaction, and the Diels-Alder reaction.
4. Applications of Organic and Inorganic Intermediate Species
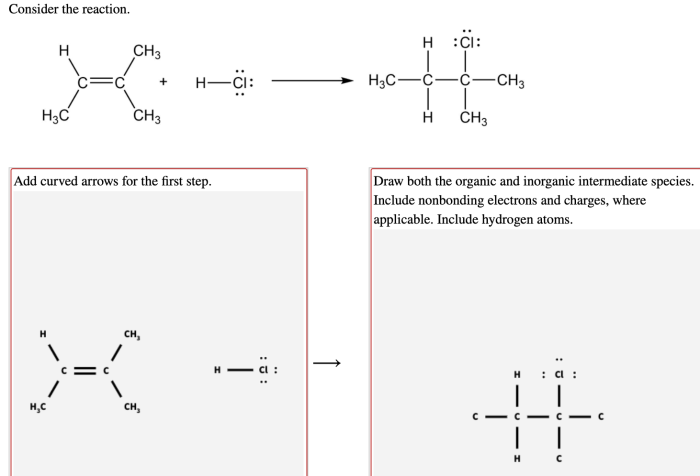
4.1. Applications of Organic and Inorganic Intermediate Species in Various Fields
Organic and inorganic intermediate species have a wide range of applications in various fields, including the synthesis of pharmaceuticals, materials, and other products.
4.2. Use of Organic and Inorganic Intermediate Species in the Synthesis of Pharmaceuticals, Materials, and Other Products
Organic and inorganic intermediate species are used in the synthesis of a wide range of products, including pharmaceuticals, materials, and other products. For example, carbocations are used in the synthesis of many pharmaceuticals, and metal complexes are used in the synthesis of many materials.
4.3. Examples of Products that are Made Using Organic and Inorganic Intermediate Species
Some examples of products that are made using organic and inorganic intermediate species include pharmaceuticals, plastics, and fuels.
FAQ Overview
What are the key differences between organic and inorganic intermediate species?
Organic intermediate species contain carbon atoms, while inorganic intermediate species do not. Organic species are typically more reactive and less stable than inorganic species.
How are organic and inorganic intermediate species formed?
Organic intermediate species are often formed through bond breaking and rearrangement reactions, while inorganic intermediate species can be formed through a variety of mechanisms, including redox reactions and ligand exchange.
What is the role of organic and inorganic intermediate species in chemical reactions?
Intermediate species play a crucial role in chemical reactions by providing a pathway for reactants to transform into products. They can stabilize high-energy transition states and lower the activation energy of reactions.